Serialisation and Verification
What does serialisation and verification of medicinal products mean?
A medicinal product pack is labelled with a product code and a randomised unique serial number (serialisation), which, together with its batch number and expiry date, is encoded in a two-dimensional barcode. Based on this procedure, the pack is uniquely identifiable throughout Europe.
Before a medicine is handed out by a pharmacy, a hospital pharmacy or a dispensing physician, the serial number of the medicinal product is verified (verification) against a data repository and after successful verification the medicinal product is decommissioned. This is to ensure the authenticity of the medicinal product. Should any inconsistencies appear during the verification process the medicine must not be handed out.

Implementation of an EU Directive
Strict requirements apply throughout the EU as of 9 February 2019: Prescription-only medicinal products must bear safety features. These safety features ensure that no falsified medicinal product enters into the legal supply chain. Directive 2011/62/EU of the European Parliament and of the Council as well as Delegated Regulation EU 2016/161 of the EU Commission form the legal basis for the implementation of this procedure. The aforementioned safety features encompass a unique identifier and a device allowing to detect tampering.
In order to record the unique identifiers each EU as well as EEA member state and Switzerland established a national repository. The national repository implemented in Austria is connected to an European Hub to ensure the verification of individual medicinal product packs also throughout Europe. This hub is operated by the European Medicines Verification Organisation (EMVO).
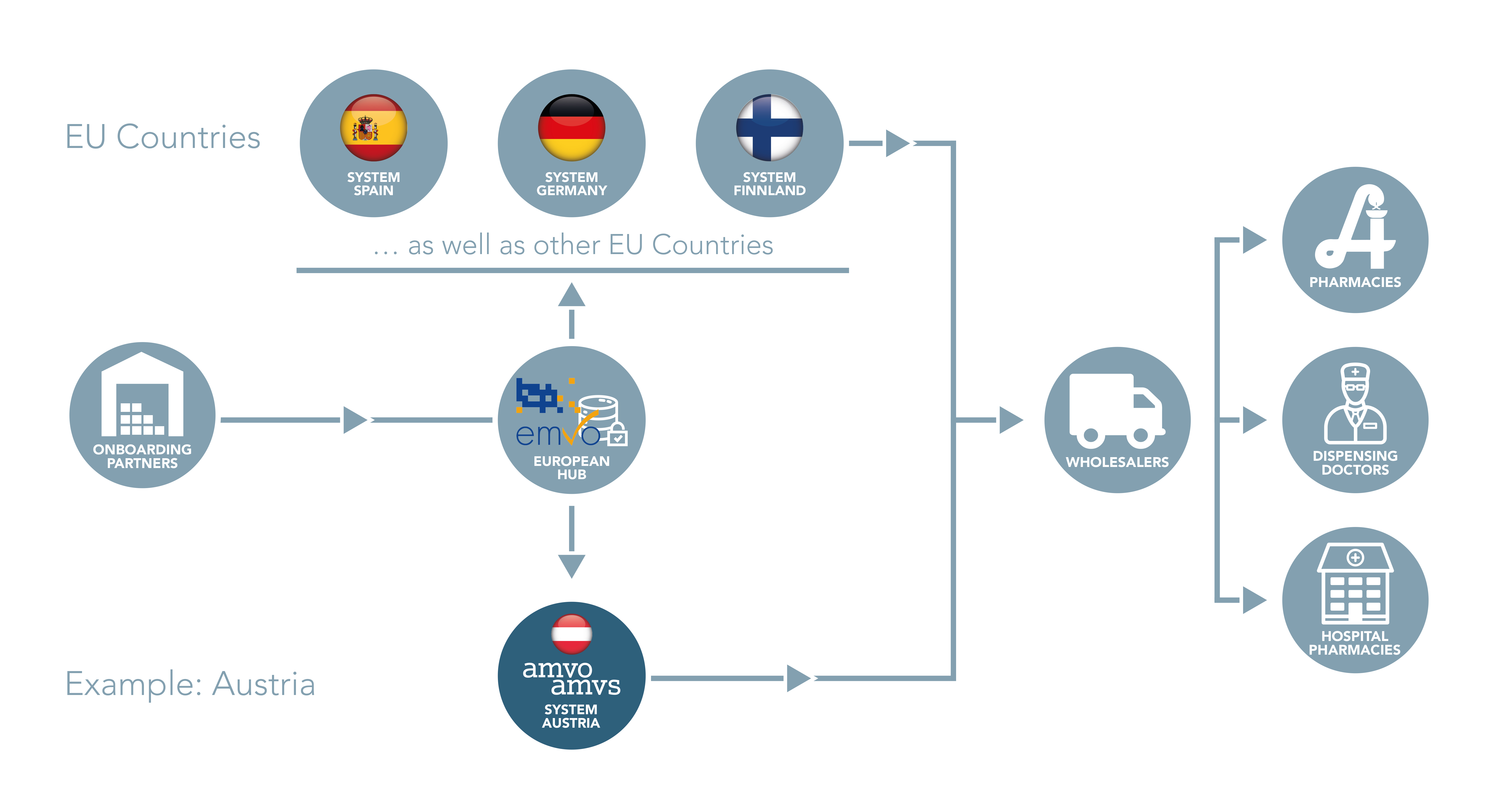
Pursuant to Directive 2011/62/EU and Delegated Regulation EU 2016/161, all parties that form part of the pharmaceutical supply chain are responsible for jointly operating the repositories system. The costs are to be borne by the pharmaceutical industry. AMVO (Austrian Medicines Verification Organisation) is responsible for implementing the system called for by the Falsified Medicines Directive. This association was formed by Pharmig, the Association of the Austrian Pharmaceutical Industry, Österreichischer Generikaverband OeGV (Austrian Generic Medicines Association), Austrian Association of Full-Line Pharmaceutical Wholesalers PHAGO, the Austrian Chamber of Pharmacists for employed and self-employed pharmacists working in public pharmacies or hospitals as well as the Austrian Medical Chamber for dispensing doctors.
AMVS GmbH, a 100% subsidiary of AMVO, is the company that operates the Austrian data repository.