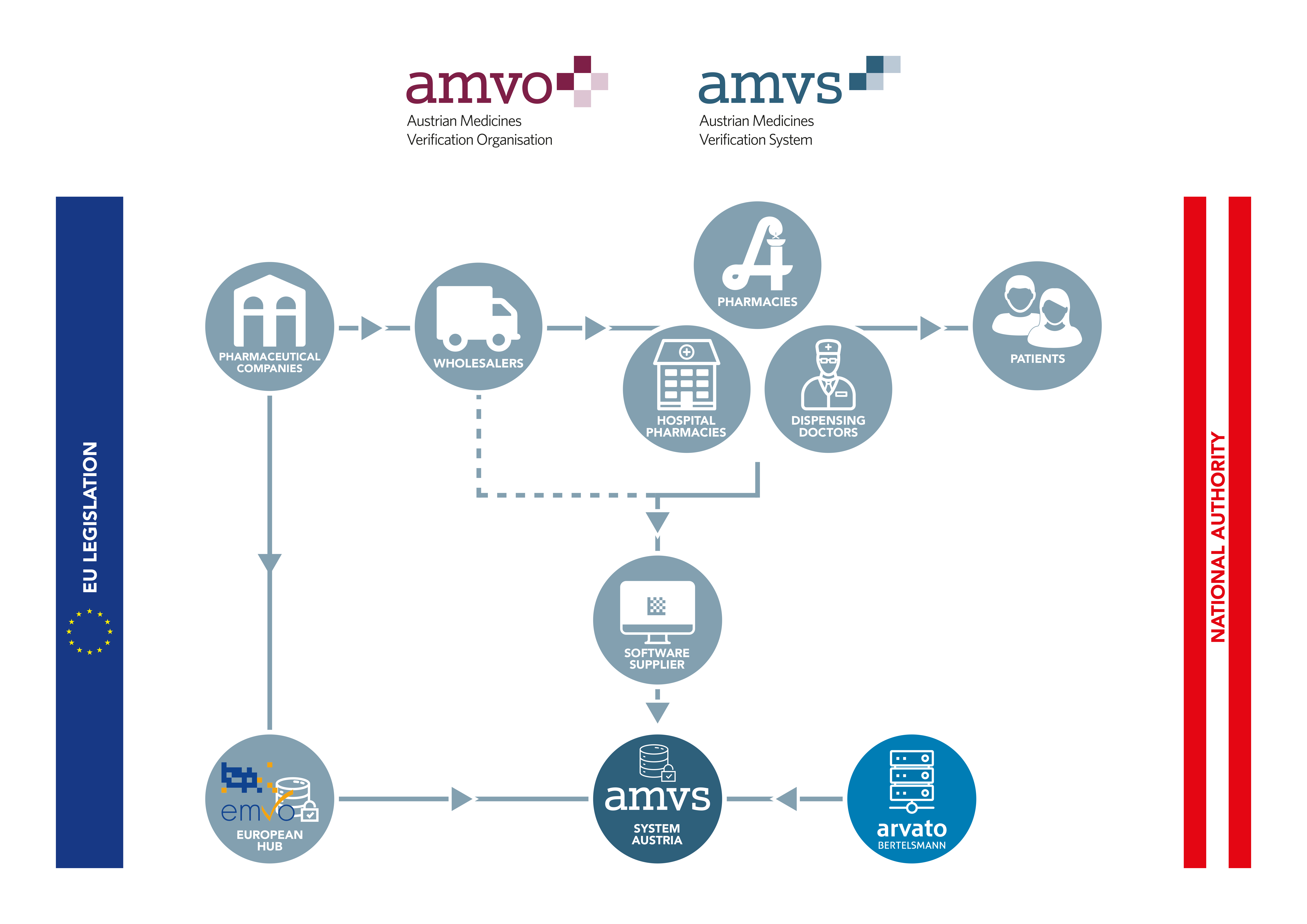
All pharmaceutical companies responsible for distributing products subject to serialisation in Austria must register with AMVS GmbH and conclude an agreement for the use of the system. By doing so, the companies agree to contribute to funding of the national verification system by paying an one-off accession fee and an ongoing annual service fee.
The pharmaceutical companies responsible for the financing of the Austrian repositories system are the pharmaceutical companies included in the list of products ("Warenverzeichnis") of the Austrian Pharmacist's Publishing House ("Österreichischer Apotheker-Verlag") as manufacturers with a manufacturer code. Those companies also are responsible for all other medicinal products of a marketing authorisation holder that are not listed in the "Warenverzeichnis".
If a marketing authorisation holder is not listed with any medicinal product in the "Warenverzeichnis", he has to conclude the agreement with AMVS GmbH himself.
A responsible pharmaceutical company fee overview can be found at this link. More detailed information can be obtained directly from AMVS GmbH.
Responsible pharmaceutical companies in Austria need to closely cooperate with the onboarding partner at EMVO as well as with marketing authorisation holders and manufacturers in order to support system implementation. The cooperation aims to properly implement national requirements (e.g.coding rules) and consequently ensures smooth processes with endusers (wholesalers, dispensing persons).
For information on participation in the Austrian system please contact office@amvs-medicines.at.
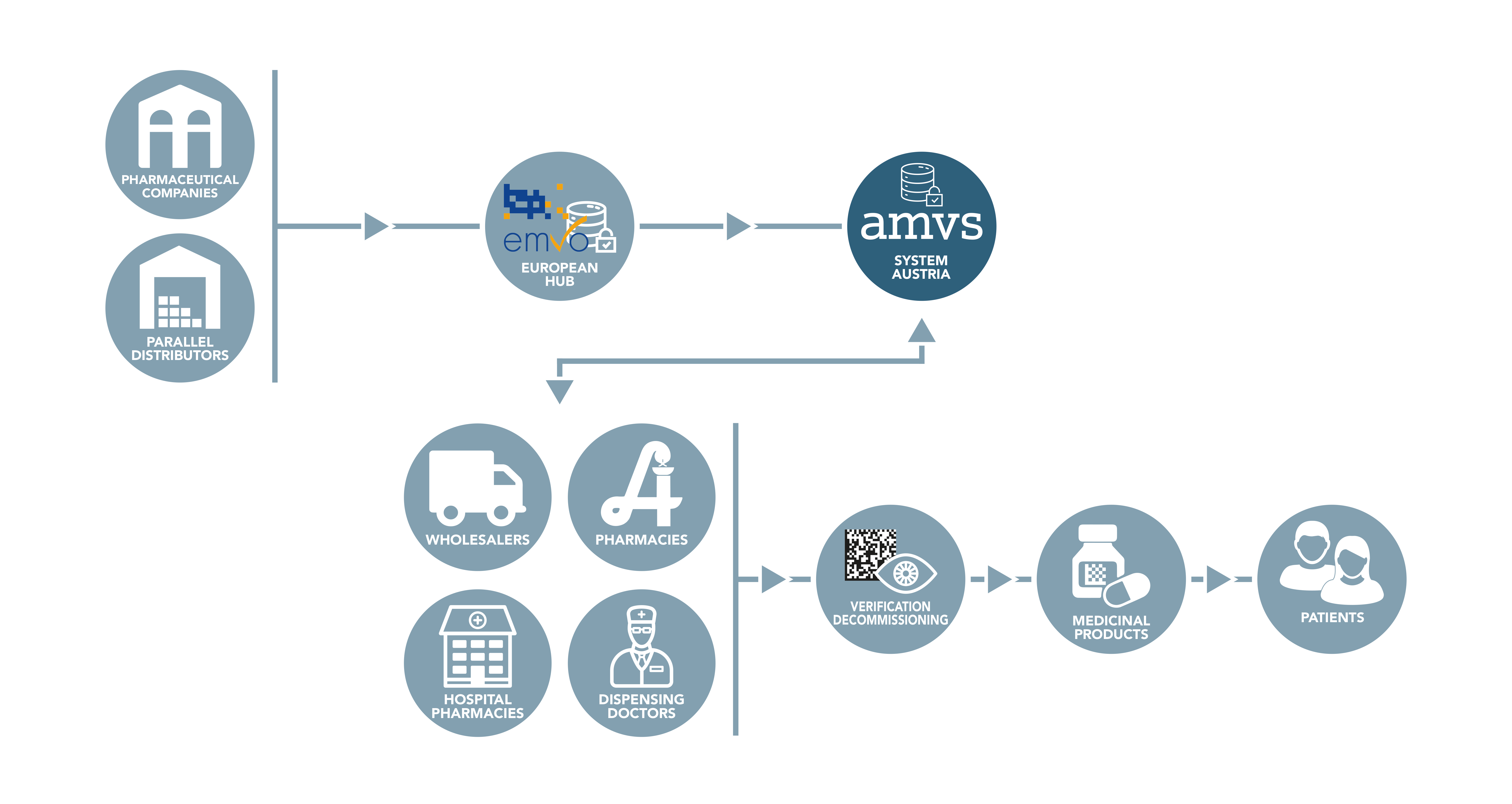
While the data from the pharmaceutical industry is uploaded to the system via the European Hub, all other parties in the supply chain are connected directly to the national systems. These are called endusers.
All Austrian companies authorised to act as wholesalers of medicinal products that perform actions according to the delegated regulation in Austria must connect to the national repository. Likewise, all public pharmacies, hospital pharmacies and dispensing doctors must connect to the Austrian system in order to be able to comply with applicable legal requirements.
To be able to participate in the national system, it is first of all necessary to adapt the software to enable linking up with the repository.
The software suppliers of the endusers must register on Arvato Systems’ SWS portal to obtain the necessary technical documentation in order to roll out the software and to connect the endusers with the system after the implementation of the software has been performed.
It is also required for all endusers to conclude an enduser agreement with AMVS GmbH. Once the agreement is signed user credentials necessary for connection are provided.
Wholesalers and dispensing persons may contact office@amvs-medicines.at to obtain further information on participation in the system.